1. How to remove nitrogen from wastewater?
Denitrification technology includes chemical and biological methods. Due to the secondary pollution generated by chemical methods and their high cost, biological denitrification technology is generally used.
1.1. Biological denitrification
The denitrification of wastewater biological treatment mainly relies on specialized bacteria to achieve the transformation of nitrogen forms. Nitrogen containing organic compounds are first decomposed and transformed into ammonia nitrogen NH4+or NH3 under the action of microorganisms, and this process is called "ammonification reaction". Nitrifying bacteria convert ammonia nitrogen into nitrate, a process known as nitrification reaction; Denitrifying bacteria convert nitrate into nitrogen, a reaction known as denitrification; Nitrogen containing organic compounds are ultimately converted into nitrogen and removed from wastewater.
(1) Nitrification process
The process by which nitrifying bacteria convert ammonia nitrogen into nitrate is called nitrification, which is a two-step process that utilizes two types of microorganisms - nitrite bacteria and nitrate bacteria. These two types of bacteria are collectively referred to as nitrifying bacteria, which utilize inorganic carbon sources such as CO32-, HCO3-, and CO2.
The first step is for nitrite bacteria to convert ammonia nitrogen into nitrite, and the second step is for nitrate bacteria to convert nitrite into nitrate. These two processes release energy, and nitrifying bacteria use this energy to synthesize new cells and maintain normal life activities. The conversion of ammonia nitrogen to nitrate nitrogen does not remove nitrogen but reduces its oxygen demand. Oxidizing 1g of ammonia nitrogen requires approximately 4.3g of O2 and 8.64g of HCO3- (equivalent to 7.14g of CaCO3 alkalinity).
The influencing factors of nitrification process:
① Temperature: The most suitable temperature range for nitrification reaction is 30-35 ℃. Temperature not only affects the specific growth rate of nitrifying bacteria, but also affects their activity.
② Dissolved oxygen: The nitrification reaction must be carried out under aerobic conditions, with a dissolved oxygen concentration of 0.5~0.7mg/L being the limit that nitrifying bacteria can tolerate. When the dissolved oxygen is below 2mg/L, nitrogen may be completely nitrified, but it requires a longer sludge retention time. Therefore, the dissolved oxygen concentration of the mixed solution should generally be maintained above 2mg/L.
③ PH and alkalinity: Nitrobacteria are particularly sensitive to pH, and the optimal pH for nitrification reaction is between 7.2 and 8. Approximately 7.14g of CaCO3 alkalinity is required for every 1g of ammonia nitrogen to be nitrated. If the wastewater does not have sufficient alkalinity for buffering, the nitrification reaction will lead to a decrease in pH value and a slower reaction rate.
④ Toxic substances: Excessive ammonia nitrogen, heavy metals, toxic substances, and certain organic substances all have inhibitory effects on nitrification reactions.
⑤ Mud age: Generally speaking, the mud age of the system should be more than twice the generation cycle of nitrifying bacteria, generally not less than 3-5 days. In winter, when the water temperature is low, a longer mud age is required. To ensure sufficient nitrification reaction throughout the year, the mud age is usually greater than 10 days.
⑥ Carbon nitrogen ratio: The ratio of BOD5 to TKN is C/N, which reflects the ability of heterotrophic bacteria and nitrifying bacteria to compete for substrates and dissolved oxygen in activated sludge systems. The difference in C/N directly affects the denitrification effect. It is generally believed that when the BOD5 load of the treatment system is lower than 0.15 BOD5/(MLVSS·d), the nitrification reaction can proceed normally.
(2) Denitrification process
The denitrification process is the process by which denitrifying bacteria differentiate nitrate, that is, the nitrate and nitrite produced by the denitrifying bacteria are reduced to nitrogen by the denitrifying bacteria and then overflow from the water.
The denitrification process is mainly carried out under anaerobic conditions, and the concentration of dissolved oxygen cannot exceed 0.2mg/L, otherwise the denitrification process will stop.
Denitrification is also divided into two steps. The first step is to convert nitrate into nitrite, and the second step is to convert nitrite into nitric oxide, nitrous oxide, and nitrogen gas.
Factors affecting denitrification:
① Temperature: The optimal temperature range for denitrification is 35-45℃.
② Dissolved oxygen: In order to ensure the progress of denitrification, it is necessary to maintain a strict anaerobic state and maintain an oxidation-reduction potential of -50~-110mV; To ensure the normal progress of denitrification reaction, the dissolved oxygen in the suspended activated sludge system is kept below 0.2mg/L; Attached biological treatment systems can allow for higher dissolved oxygen concentrations, generally below 1mg/L.
③ PH value: The optimal range is between 6.5 and 7.5.
④ Carbon source organic matter: Sufficient carbon source needs to be provided, and the denitrification rate varies depending on the carbon source material.
⑤ Carbon to nitrogen ratio: Theoretically, converting 1g of nitrate nitrogen into nitrogen requires 2.86g of carbon source material BOD5.
Many people are unclear about how the number 2.86 was derived. By the way, I would like to mention (citing an expert's explanation here):
When we talk about C, most of the time we refer to COD (Chemical Oxygen Demand), which means C/N is actually COD/N. COD is a method of measuring organic matter content using oxygen demand, as shown in equation (1) for the process of methanol oxidation. The two are not the same, but they increase proportionally, and the more organic matter there is, the more oxygen demand there is. Therefore, we can use COD to characterize the changes in organic matter. CH3OH+1.5O2 → CO2+2H2O (1)
a. When denitrification is carried out, if the growth of microorganisms themselves is not included, the equation is very simple, usually represented by methanol as the carbon source.
6NO3-+5CH3OH → 3N2+5CO2+7H2O+6OH - (2) The corresponding relationship between methanol and oxygen (i.e. COD) can be obtained from equation (1): 1mol of methanol corresponds to 1.5mol of oxygen, and the corresponding relationship between methanol and NO3- can be obtained from equation (2). 1mol of methanol corresponds to 1.2molNO3-. Comparing the two, 1mol of NO3- N corresponds to 1.25molO2, that is, 14gN corresponds to 40gO2, so C/N=40/14=2.86.
b. When denitrification is carried out, if it includes the growth of microorganisms themselves, as shown in equation (3).
NO3-+1.08CH3OH → 0065C5H7NO2+0.47N2+1.68CO2+HCO3- (3)
Similarly, we can calculate C/N=3.70.
c. Note: The original calculation has been completed here, but I would like to illustrate the first scenario. The following calculation is only a mathematical calculation of a chemical equation and does not necessarily mean that such a reaction has actually occurred. If we sort out equations (1) and (2), N2+2.5O2+2OH - → 2NO3-+H2O, which are inconvenient for negative ions, we subtract 2OH - from both sides, N2+2.5O2 → N2O5. Among them, N is derived from NO3-, and O can represent organic matter. Therefore, the theoretical carbon source demand for denitrification without microbial growth is actually equivalent to the oxygen demand for oxidizing N2 into N2O5, which is the mass ratio of O/N in N2O5 molecules. This makes it even simpler. C/N=16×5/(14×2)=20/7=2.86. It can be inferred that the theoretical C/N ratio for pure denitrification of NO2-- N is the mass ratio of O/N in N2O3 molecule=16×3/(14×2)=12/7=1.71.
⑥ Toxic substances: When the nickel concentration exceeds 0.5mg/L, the nitrite content exceeds 30mg/L, or the salt concentration exceeds 0.63%, denitrification will be inhibited.
(3) Basic conditions for biological denitrification
① Nitrate: The generation and presence of nitrate is a prerequisite for denitrification, and nitrogen-containing organic compounds such as proteins, amino acids, urea, lipids, nitro compounds, etc. in wastewater must be converted into nitrate nitrogen first.
② No dissolved oxygen: The oxygen in the reactor will be preferentially utilized by the organism, thereby reducing the amount of nitrite that the reactor can remove. When the dissolved oxygen exceeds 0.2mg/L, there is no significant denitrification effect.
③ Facultative microbiota: In most cases, bacteria generally have denitrification habits, and the microbial denitrification in wastewater treatment alternates repeatedly under aerobic and anaerobic conditions, with facultative microbiota being the main type.
④ Electronic donor: The energy for biological denitrification comes from the carbon organic matter that acts as an electron donor during the denitrification process. During denitrification, the organic matter in the wastewater must be sufficient, otherwise external carbon sources such as methanol, ethanol, and acetic acid need to be added.
(4) Biological denitrification treatment method for wastewater
Biological denitrification process is a single-stage or multi-stage activated sludge system that includes nitrification and denitrification processes. From the perspective of reactors that complete biological nitrification, denitrification processes can be divided into two categories: microbial suspension growth type (activated sludge method and its deformation) and microbial attachment growth type (biofilm reactor).
The multi-stage activated sludge system has a multi-stage sludge reflux system, which is a traditional biological denitrification method that involves separate nitrification and denitrification. This process can achieve quite good BOD5 removal and denitrification effects, but its disadvantages include long process, multiple structures, high infrastructure costs, the need for additional carbon sources, high operating costs, and a certain amount of residual methanol in the effluent. The single-stage activated sludge system, on the other hand, realizes the oxidation, nitrification, and denitrification of carbon containing organic matter in one activated sludge system, with only one sedimentation tank, that is, a sludge reflux system.
The representative methods for single-stage activated sludge denitrification system are anaerobic/aerobic (A/O) process and four stage Bardenpho process (A/O/A/O). Other methods include anaerobic/anaerobic/aerobic (A2/O) process, Phoredox (five stage Bardenpho) process, UCT process, VIP process, etc;
In addition, when the oxidation ditch, SBR method, and circulating activated sludge method have denitrification function by adjusting the operating mode, they are also classified as single-stage activated sludge denitrification systems. Among them, A2/O process, Bardenpho process, Phoredox process, UCT process, VIP process, etc. all have denitrification and phosphorus removal functions.
The biofilm reactor is suitable for the growth of nitrifying bacteria with long generation cycles, and the immobilized microorganisms in it provide suitable growth environments for both nitrifying and denitrifying bacteria. Therefore, in general biofilm reactors, nitrification and denitrification processes also exist simultaneously.
In the existing activated sludge treatment process, the addition of carriers such as powdered activated carbon can not only improve the ability to remove BOD5, but also enhance the nitrification and denitrification efficiency of the entire system. If the wastewater that has already been nitrified is refluxed into anaerobic biofilm reactors such as low-speed rotating biological turntables and biofilters with lower air blowing rates, better denitrification results can be achieved without the need for sludge reflux.
1.2. Chemical denitrification
Wastewater with a mass concentration of ammonia nitrogen greater than 500mg/L is called high concentration ammonia nitrogen wastewater. The content of ammonia nitrogen in industrial wastewater and urban domestic sewage has sharply increased, showing the characteristics of multiple sources of ammonia nitrogen pollution, large emissions, and increased concentration of emissions. The treatment technologies for high ammonia nitrogen wastewater mainly use methods such as blow off and chemical precipitation.
(1) Blow off method
The process of introducing air into wastewater to transfer soluble gases and volatile solutes from the liquid phase to the gas phase, and treating wastewater is called blowing off. The common process flow is shown in Figure 1.
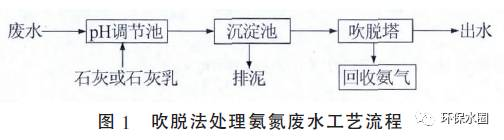
The basic principles of the stripping method are gas-liquid phase equilibrium and mass transfer velocity theory. Adjust the pH of ammonia nitrogen wastewater to alkaline. At this time, ammonium ions are converted into ammonia molecules, and then gas is introduced into the water to fully contact the liquid. The dissolved gas and volatile ammonia molecules in the wastewater pass through the gas-liquid interface and transfer to the gas phase, thereby achieving the purpose of removing ammonia nitrogen. Air or water vapor is commonly used as the carrier gas, the former is called air stripping, and the latter is called steam stripping.
The steam stripping method has a high efficiency, with an ammonia nitrogen removal rate of over 90%. However, it consumes a lot of energy and is generally used in industries such as steelmaking, fertilizer, and petrochemicals. Its advantage is that ammonia can be recycled and reused, and after stripping treatment, ammonia water with an ammonia mass fraction of over 30% can be recovered.
Although the efficiency of air stripping method is lower than that of steam method, it has low energy consumption, simple equipment, and convenient operation. When the total amount of ammonia nitrogen is not high, using air stripping method is more economical. At the same time, sulfuric acid can be used as an absorbent to absorb the ammonia nitrogen blown out, and the generated ammonium sulfate can be made into fertilizer.
However, in the large-scale production process of ammonia stripping and stripping towers, the generation of scale is a challenging problem. Installing a spray water system can effectively solve the problem of soft scale, but for hard scale, the spray device cannot eliminate it. In addition, at low temperatures, the removal rate of ammonia nitrogen is low, and the blown off gas forms secondary pollution. Therefore, the stripping method is generally used in conjunction with other ammonia nitrogen wastewater treatment methods to pretreat high concentration ammonia nitrogen wastewater.
The optimal blowing process conditions are shown in Table 1.
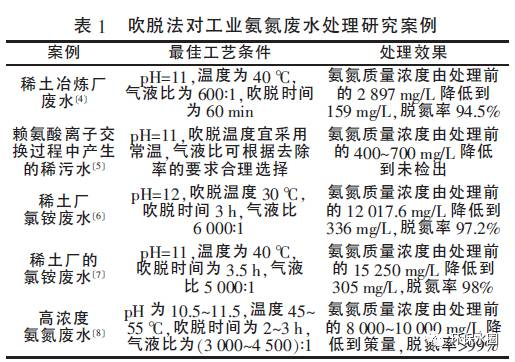
By comparing and analyzing Table 1, it can be concluded that:
① The generally suitable pH for blow off method is around 11;
② Considering economic factors, temperatures around 30-40 ℃ are more feasible and have a high processing rate;
③ The blowing time is about 3 hours;
④ The gas-liquid ratio is around 5000:1, and the higher the stripping temperature, the smaller the gas-liquid ratio;
⑤ The concentration of wastewater after blowing can be reduced to a medium to low concentration;
⑥ The denitrification rate remains above 90%. Although the stripping method can remove most of the ammonia nitrogen, the ammonia nitrogen in the treated wastewater is still above 100 mg/L, which cannot be directly discharged and requires further advanced treatment.
(2) Chemical precipitation method (ammonium magnesium phosphate precipitation method)
The principle of chemical precipitation method is to add agents containing Mg2+and PO43- to ammonia nitrogen wastewater, so that the ammonia nitrogen and phosphorus in the wastewater are precipitated in the form of bird droppings (ammonium magnesium phosphate), and the nitrogen and phosphorus in the wastewater are recovered at the same time.
The advantages of chemical precipitation method mainly lie in: relatively simple process design and operation; Stable response, less affected by external environment, strong shock resistance; The denitrification rate is high and the effect is obvious. The generated magnesium ammonium phosphate can be used as inorganic composite fertilizer, solving the problems of nitrogen recovery and secondary pollution, and has good economic and environmental benefits. The ammonium magnesium phosphate precipitation method is suitable for treating industrial wastewater with high ammonia nitrogen concentration.
Table 2 summarizes some cases of using chemical precipitation method to treat ammonia nitrogen wastewater.
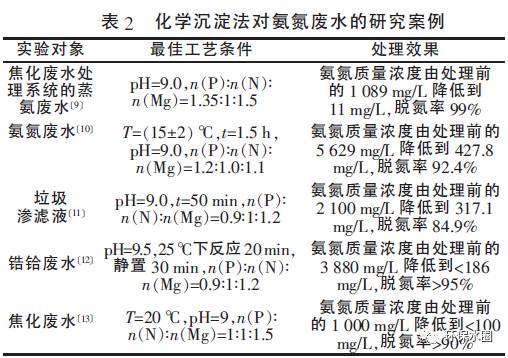
It can be seen that the suitable conditions for treating ammonia nitrogen wastewater by the ammonium magnesium phosphate precipitation method are: pH is about 9.0, n (P): n (N): n (Mg) is around 1:1:1.2, and the denitrification rate of the ammonium magnesium phosphate precipitation method can be maintained at a high level, generally reaching over 90%.
1.3. Low concentration ammonia nitrogen industrial wastewater treatment technology
There are two main types of ammonia nitrogen in wastewater, one is ammonia nitrogen formed by ammonia water, and the other is ammonia nitrogen formed by inorganic ammonia, mainly ammonium sulfate, ammonium chloride, etc. Ammonia nitrogen is one of the important factors causing eutrophication in water bodies. When recycling and reusing such wastewater, it can also cause corrosion to metals in pipelines, shorten the lifespan of equipment and pipelines, and increase maintenance costs. At present, the technologies commonly used in industry to treat low concentration ammonia nitrogen include adsorption, point chlorination, biological method, membrane technology, etc.
① Adsorption method
Adsorption is the process in which the concentration of one or several substances (called adsorbents) automatically changes on the surface of another substance (called adsorbents). Its essence is a mass transfer phenomenon of substances from the liquid or gas phase to the solid surface.
Adsorption method is one of the promising methods for treating low concentration ammonia nitrogen wastewater. The adsorption method often uses porous solids as adsorbents, which can be divided into physical adsorption, chemical adsorption, and exchange adsorption according to different adsorption principles. The ideal method for treating low concentration ammonia nitrogen wastewater is ion exchange adsorption, which belongs to a type of exchange adsorption method. It uses exchangeable ions on the adsorbent to exchange with NH4+in the wastewater and adsorb NH3 molecules to achieve the removal of ammonia in the water. This is a reversible process, and the concentration difference between ions and the affinity of the adsorbent for ions provide power for the adsorption process.
Commonly used adsorbents with good adsorption performance include zeolite, activated carbon, coal, ion exchange resin, etc. Depending on their adsorption principles, these adsorbent materials have different adsorption effects on different adsorbents.
This method is generally only applicable to low concentration ammonia nitrogen wastewater, while for high concentration ammonia nitrogen wastewater, the use of adsorption method may cause operational difficulties due to frequent replacement of adsorbents. Therefore, it is necessary to combine other processes to collaboratively complete the denitrification process. There are many adsorbents available for the adsorption method, but the adsorption capacity of ammonia nitrogen in wastewater varies greatly among different adsorbents. Table 3 compares the adsorption effects of some adsorbents.
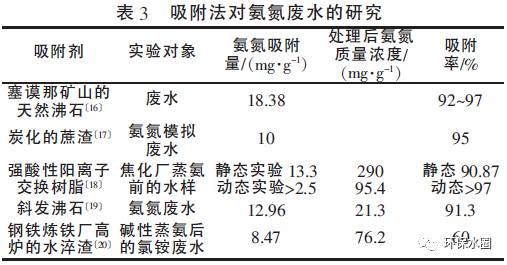
From Table 3, it can be seen that for traditional adsorbents such as zeolite and exchange resin, their treatment rate for ammonia nitrogen is relatively high, generally reaching over 90%.
② Fold point chlorination method
The breakpoint chlorination method is a commonly used denitrification process in sewage treatment engineering. Its principle is to introduce chlorine gas into ammonia nitrogen wastewater to reach a critical point, which oxidizes ammonia nitrogen into nitrogen. The reaction equation is: NH4+1.5HOCl → 0.5N2+1.5H2O+2.5H++1.5Cl-
The advantages of the breakpoint chlorination method are: high treatment efficiency and stable effect, with a removal rate of up to 100%; This method is not affected by salt content or water temperature, and is easy to operate; The lower the organic matter content, the better the ammonia nitrogen treatment effect, and there is no precipitation; Low initial investment, quick and complete response; It can have a sterilizing and disinfecting effect on water bodies.
However, the breakpoint chlorination method is only suitable for the treatment of low concentration wastewater, so it is mostly used for the deep treatment of ammonia nitrogen wastewater. The disadvantages of this method are: high consumption of liquid chlorine, high cost, and high safety requirements for the storage and use of liquid chlorine. The reaction byproducts chloramine and chlorinated organic compounds can cause secondary pollution to the environment.
1.4. Comparison of Treatment Methods for Industrial Ammonia Nitrogen Wastewater with Different Concentrations

2. How to remove phosphorus from wastewater?
The conventional biological treatment method can remove some phosphorus from wastewater by discharging and treating residual sludge. Some special processes or ordinary processes with phosphorus removal function after adjusting the operating mode can achieve better phosphorus removal effects. Specific methods include A/O, A ²/ O. SBR, oxidation ditch, etc. However, the phosphorus removal effect of biological treatment methods is limited. When the phosphorus emission standards are high, chemical phosphorus removal or a combination of biological and chemical phosphorus removal methods are often required. Chemical phosphorus removal is the process of adding chemical agents to water to generate insoluble phosphates, which are then removed from wastewater using methods such as sedimentation, air flotation, or filtration. The commonly used pesticides for chemical phosphorus removal include lime, aluminum salts, and iron salts.
2.1. Chemical phosphorus removal
(1) Lime phosphorus removal
Lime phosphorus removal is the reaction between adding lime and phosphate to produce hydroxyapatite precipitation. Due to the fact that after lime enters water, it first reacts with the alkalinity of the water to form calcium carbonate precipitate, and then excess calcium ions can react with phosphate to form hydroxyapatite precipitate, the required amount of lime mainly depends on the alkalinity of the wastewater to be treated, rather than the phosphate content of the wastewater. In addition, the magnesium hardness of wastewater is also a factor affecting lime phosphorus removal. Because under high pH conditions, the generated Mg (OH) 2 precipitate is a colloidal precipitate, which not only consumes lime but also is not conducive to sludge dewatering. The pH has a significant impact on lime phosphorus removal. As the pH increases, the solubility of hydroxyapatite sharply decreases, indicating an increase in phosphorus removal rate. When the pH exceeds 9.5, all phosphates in the water become insoluble precipitates. The best phosphorus removal effect is generally achieved by controlling the pH between 9.5 and 10. The amount of lime added to different wastewater should be determined through experiments.
There are three specific methods for removing phosphorus with lime. One is to add lime before the initial sedimentation tank of the sewage treatment plant, but to the secondary sedimentation tank after the biological treatment of the sewage. The third is to add lime after the biological treatment system and equipped with a re carbonation system.
(2) Aluminum salt phosphorus removal
The commonly used reagents for aluminum salt phosphorus removal are aluminum sulfate and sodium aluminate. The difference is that adding aluminum sulfate will lower the pH of the wastewater, while adding sodium aluminate will increase the pH of the wastewater. Therefore, aluminum sulfate and sodium aluminate are suitable for treating alkaline and acidic wastewater, respectively. The addition of aluminum salts is relatively flexible, which can be added before the initial sedimentation tank, in the aeration tank, or between the aeration tank and the secondary sedimentation tank. Chemical phosphorus removal and biological treatment systems can also be separated, and aluminum salts can be added to the effluent of the secondary sedimentation tank for coagulation filtration, or aluminum salts can be added before the filter tank for micro flocculation filtration. Adding before the initial sedimentation tank can improve the removal rate of organic matter and SS in the initial sedimentation tank. Adding between the aeration tank and the secondary sedimentation tank can improve the mixing effect of the agent due to turbulence in the channel or pipeline. Adding after the biological treatment system can improve the phosphorus removal effect due to the hydrolysis effect of biological treatment on phosphorus. Due to the influence of wastewater alkalinity and organic matter, the chemical reaction for phosphorus removal is a complex process. Therefore, the optimal dosage of aluminum salts cannot be determined by calculation and must be determined through experiments.
(3) Iron salt phosphorus removal
Iron trichloride, ferrous chloride, ferrous sulfate, and iron sulfate can all be used for phosphorus removal, with ferric chloride being commonly used.
Similar to aluminum salts, a large amount of ferric chloride needs to meet the Fe (OH) 3 generated by the reaction with alkalinity to promote the precipitation and separation of colloidal iron phosphate. The optimal pH range for iron phosphate precipitation is 4.5~5.0. In practical applications, pH values around 7 or even above 7 still have good phosphorus removal effects.
Adding approximately 45-90mg/L ferric chloride to urban wastewater can remove 85% to 90% of phosphorus. Like aluminum salts, the addition point of iron salts can be in the pre-treatment, secondary treatment, or tertiary treatment stages.
However, chemical phosphorus removal can cause some problems:
① The biggest problem with chemical phosphorus removal is that it significantly increases the amount of sludge in wastewater treatment.
Because the metal phosphates and metal hydroxides produced during phosphorus removal exist in the form of suspended solids in water, ultimately turning into sludge. Adding metal salts before the initial sedimentation tank increases the sludge volume by 60% to 100%, resulting in a 60% to 70% increase in the entire sewage treatment plant sludge volume. Adding metal salts during the secondary treatment process increases the remaining sludge volume by 35% to 45%
② Chemical phosphorus removal can reduce sludge concentration by about 20%, resulting in an increase in sludge volume, which increases the difficulty of sludge treatment and disposal.
③ When using chemical phosphorus removal, the soluble solid content in the effluent increases. If the solid-liquid separation is not good, iron salt phosphorus removal will cause the effluent to turn slightly red.
2.2. Biological phosphorus removal
(1) The principle of biological phosphorus removal
The principle of biological phosphorus removal in wastewater is to artificially create a biological excess phosphorus removal process to achieve controllable phosphorus removal effects. The entire process must utilize anaerobic microorganisms to achieve biological phosphorus removal by creating anaerobic links.
① Phosphorus release under anaerobic conditions
In the absence of dissolved oxygen or nitrate nitrogen, facultative bacteria convert soluble BOD5 into low molecular volatile organic acid VFA through fermentation. Phosphorus accumulating bacteria absorb these fermentation products or VFAs from raw wastewater and transport them into cells, assimilating them into intracellular carbon energy storage material PHB. The required ability comes from the hydrolysis of polyphosphate and the fermentation of intracellular sugars, leading to the release of phosphate.
② Phosphorus uptake under aerobic conditions
Under aerobic conditions, the activity of polyphosphate accumulating bacteria is restored and stored in the form of polyphosphate, exceeding the required amount of phosphorus for growth. Energy is generated through the oxidation metabolism of PHB, which is used for phosphorus absorption and polyphosphate synthesis. The energy is captured and stored in the form of polyphosphate high-energy bonds, and phosphate is removed from water.
③ Discharge of phosphorus rich sludge
The generated phosphorus rich sludge is discharged in the form of residual sludge to remove phosphorus. From an energy perspective, phosphorus accumulating bacteria release phosphorus under anaerobic conditions to obtain energy and absorb dissolved organic matter in wastewater. In aerobic conditions, they degrade and absorb dissolved organic matter to obtain energy and absorb phosphorus. The key to phosphorus removal is the setting of anaerobic zones, which can be said to be the biological selector for phosphorus accumulating bacteria. Phosphorus accumulating bacteria can compete under brief anaerobic conditions, as non phosphorus accumulating bacteria absorb low molecular weight substrates and quickly assimilate and store these fermentation products, i.e. the anaerobic zone provides a competitive advantage for phosphorus accumulating bacteria. In this way, phosphorus accumulating bacteria that can absorb a large amount of phosphorus can selectively proliferate in the treatment system and achieve phosphorus removal by removing excess sludge with high phosphorus content. Another benefit of this selective proliferation is that it inhibits the proliferation of filamentous bacteria, avoiding the possibility of producing sludge with poor sedimentation performance. Therefore, anaerobic/aerobic biological phosphorus removal processes generally do not cause sludge bulking.
(2) The influencing factors of biological phosphorus removal
① Dissolved oxygen
Firstly, it is necessary to strictly control the anaerobic environment in the anaerobic zone, which directly affects the growth status, phosphorus release ability, and ability to synthesize PHB using organic substrates of phosphorus accumulating bacteria. Secondly, it is necessary to provide sufficient dissolved oxygen in the aerobic zone to meet the degradation of stored PHB by polyphosphate accumulating bacteria, releasing sufficient energy for excessive phosphorus uptake. The DO in the anaerobic section should be strictly controlled below 0.2mg/L, while the DO in the aerobic section should be strictly controlled above 2mg/L.
② Nitrate nitrogen in anaerobic zone
Nitrate nitrogen includes nitrate and nitrite. The presence of nitrate nitrogen also consumes organic matrix and inhibits the release of phosphorus by polyphosphate accumulating bacteria, thereby affecting the absorption of phosphorus by polyphosphate accumulating bacteria under aerobic conditions. In addition, the presence of nitrate nitrogen can be partially denitrified by polyphosphate accumulating bacteria as electron acceptors, without affecting their ability to use fermentation products as electron acceptors for acid production, inhibit the release and uptake of phosphorus by polyphosphate accumulating bacteria, and their ability to synthesize PHB.
③ Temperature
Generally speaking, good phosphorus removal effects can be achieved within the range of 5-30 ℃.
④ PH value
The release of phosphorus is relatively stable within the pH range of 6-8.
⑤ BOD load and organic matter properties
It is generally believed that the BOD5/TP in the influent should be greater than 15 to ensure that the phosphorus accumulating bacteria have sufficient substrate and achieve ideal phosphorus removal effects. To achieve this, partial inflow and crossing of the initial sedimentation tank can be used to obtain the required amount of BOD5 for phosphorus removal.
⑥ Mud age
The sludge age of biological treatment systems aimed at phosphorus removal is generally controlled between 3.5 and 7 days.
(3) The method of biological phosphorus removal from wastewater
Biological phosphorus removal from wastewater includes two processes: anaerobic phosphorus release and aerobic phosphorus uptake. Therefore, the process flow of biological phosphorus removal from wastewater consists of two parts: anaerobic and aerobic. According to the final removal method of phosphorus and the composition of the structure, the phosphorus removal process can be divided into mainstream phosphorus removal process and side process phosphorus removal process.
The anaerobic section of the mainstream phosphorus removal process treats wastewater in the direction of water flow, and the final removal of phosphorus is achieved through the discharge of residual sludge. Typical methods include anaerobic/aerobic (A/O) process, while other methods include anaerobic/anaerobic/aerobic (A/ ² O) Process, Phoredox process, UTC process, VIP process, SBR process, oxidation ditch process, etc.
The anaerobic section of the side flow process is not in the direction of sewage treatment flow, but in the side flow of the reflux sludge. The specific method is to divert some phosphorus containing reflux sludge to the anaerobic section to release phosphorus, and then use lime precipitation to remove phosphorus from the phosphorus rich supernatant.
(4) Notes on the operation and management of phosphorus removal facilities
① The anaerobic section is the most critical step in biological phosphorus removal, and its volume is generally determined by a hydraulic retention time of 0.5~2 hours. If the organic matter content that is easily biodegradable in the influent is high, efforts should be made to reduce the hydraulic retention time to ensure the BOD5 content in the aerobic section influent.
② If the phosphorus emission standard is high and the selected phosphorus removal process cannot meet the effluent requirements, chemical phosphorus removal or filtration treatment can be added to remove low residual phosphorus in the water.
③ The mechanism of biological phosphorus removal process is to transfer dissolution to activated sludge biological cells and remove it from the system through the discharge of residual sludge. In the process of sludge treatment, if anaerobic conditions occur, the phosphorus in the remaining sludge will be released again.
Gravity concentration is prone to anaerobic conditions, and residual sludge treatment with phosphorus removal requirements should not use this method. Instead, non anaerobic concentration methods such as air flotation concentration, mechanical concentration, and belt gravity concentration should be used. If gravity concentration is the only option, chemical precipitation facilities must be added in the process flow to remove phosphorus from the concentrated supernatant.
④ Mud age is the main factor affecting biological denitrification and phosphorus removal, and the higher the denitrification requirements, the longer the required mud age. The longer the mud age, the more unfavorable it is for phosphorus removal. Especially when the inflow BOD5/TP is less than 20, the shorter the mud age, the better. But if the influent BOD5 is low and the growth of activated sludge is slow, it is impossible to control the sludge age too short, and chemical phosphorus removal must be carried out at this time.
Source: Environmental Water Circle
Post time: May-11-2024

